Séminaire par Sandrine Huclier, SUBATECH & GIP ARRONAX- Nantes Université & CNRS, le mardi 21 Novembre à 11h
Dans le cadre de l’animation scientifique, Sandrine Huclier, chercheuse au laboratoire SUBATECH & GIP ARRONAX, Nantes Université & CNRS, présentera une conférence intitulée “Importance of separative techniques and speciation in radiopharmaceuticals drug development” le mardi 21 novembre à 11h en salle de séminaire de l’ISA.
Résumé de la présentation:
Radiopharmaceuticals deliver a radioactive isotope to the action site, such as cancerous tissue or for neurologic diseases, either to image the tissue or to deposit a dose of ionizing radiation to kill the diseased cells. Recently radiopharmaceuticals were also introduced for theranostic purposes. The « theranostic » approach is the systematic integration of diagnostic tools into the therapeutic strategy. In this approach, a « diagnostic companion » imaging component allows the visualization of cancerous lesions before administration of the therapeutic agent targeting these same lesions. In the quest for new radionuclides, the concept of precision medicine would provide personalised adjustment of radiation characteristics to optimize efficiency of medical care or therapeutic benefit for particular groups of patients.
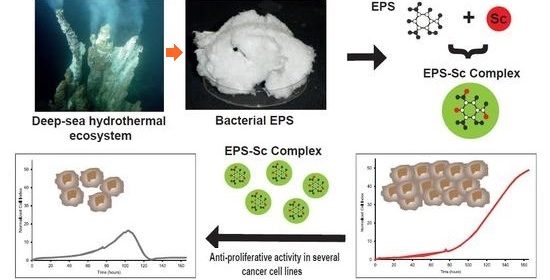
For diagnosis, radioisotope molecular imaging (PET or SPECT) offers a unique advantage in visualizing and quantifying the distribution of potential therapeutic targets throughout the body, which may be a promising approach to select patients eligible for targeted therapy and predict its efficacy. On the other hand, vectorized internal radiotherapy usually consists of the use of a specific molecule of the tumor or its microenvironment, such as an antibody, an antibody fragment or a peptide, labeled with a radionuclide (beta, alpha emitters or Auger electron emitters) and administered into the body to specifically destroy the tumor tissue. I will present some examples of theranostic using Scandium exopolysaccharide complexes developed in nuclear medicine for breast cancer, osteosarcoma and more recently for Alzheimer disease.
The second aspect of my lecture will be devoted to a nanomedicine approach with α and β- emitters together with the analytical tools developed to specifically monitor the integrity of the radiolabelled scaffolds. Metastasis is one of the major reasons for recurrence and consequent mortality in cancer and thus there is an unmet need to specifically target and cure residual disease. The short ranges of Auger electrons of less than a cell diameter makes it theoretically possible to effectively irradiate targeted cells, while largely sparing surrounding healthy tissues. I will present the development made on an Auger electron emitter generator and how understanding the speciation of the chemical elements is of utmost importance.


