Détection d’oxydation des protéines par photodissociation/spectrométrie de masse
Marion Girod (équipe AnabioMS) en collaboration avec des collègues de Croatie (Split) et de l’Institut Lumière Matière à Lyon a publié un article intitulé « Specific detection of protein carbonylation sites by 473 nm photo-dissociation mass spectrometry » dans la revue Analytical and Bioanalytical chemistry qui a fait l’objet d’un Paper in Frorefront. Cet article fait également partie de la collection spéciale « ABC Highlights: authored by Rising Stars & Top Experts »
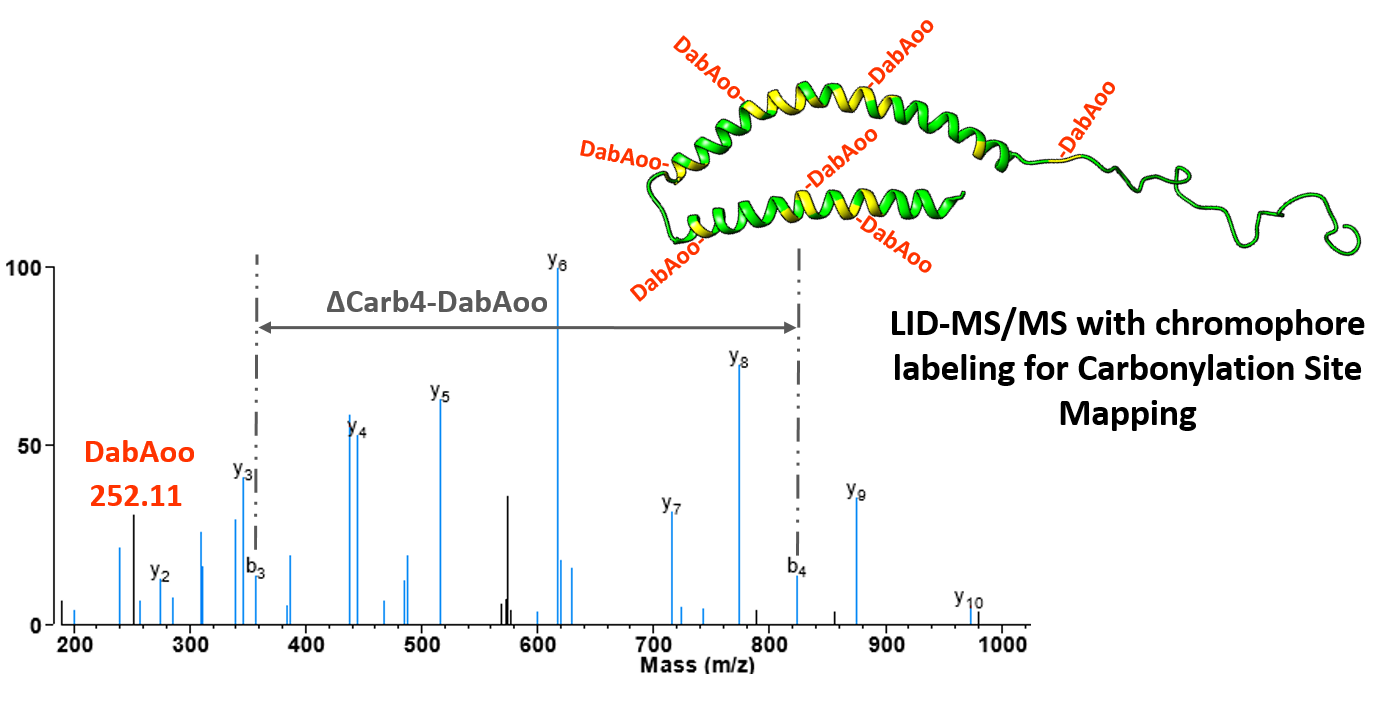
The study of protein oxidation remains a challenge despite the biomedical interest in reliable biomarkers of oxidative stress. This particularly true for carbonylations although, recently, liquid chromatography-mass spectrometry techniques (LC-MS) have been proposed to detect this non-enzymatic and poorly distributed oxidative modification of proteins using untargeted or carbonyl-reactive probe methods. These methods proved to be feasible but could not preserve the dynamic range of the protein sample, making it impossible to quantify oxidatively modified proteoforms compared with native proteoforms. Here, we propose an innovative method based on the implementation of a reactive carbonyl probe conjugated with a laser sensitive chromophore, dabcyl-aminooxy, which confers optical specificity to the LC-MS approach. In addition, our protein carbonyl detection method allows to localize individual carbonylation sites by observing fragments of derivatized oxidized peptides. Two model proteins, alpha-synuclein and beta-lactoglobulin, were oxidized and carbonylation sites were detected, resulting in the identification of respectively 34 and 77 different carbonylated amino acids. Thus, we demonstrated the application of a direct and sensitive method for studying protein carbonylation sites in complex protein extracts.


